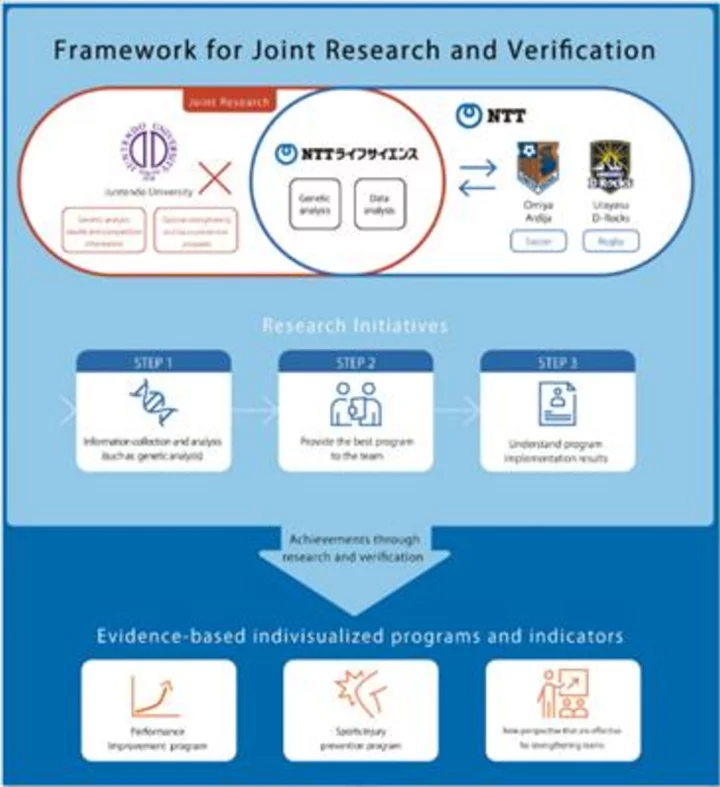
PALO ALTO, Calif.--(BUSINESS WIRE)--Aug 17, 2023--
Medable Inc., the leading technology provider for patient-centric clinical trials , today announced that it ranked in the top 8 percent (#398) and was one of the top 50 software companies on the 2023 Inc. 5000, its annual list of the fastest-growing private companies in America. Medable’s ranking was fueled by its strong three-year growth rate of 1,453% from 2019 through 2022.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230817999027/en/
Medable named #398 on Inc. 5000 (Graphic: Business Wire)
This year’s Inc. 5000 list had a median revenue growth of 219% and the companies collectively created nearly two million jobs and $358 billion in 2022 revenue. Facebook, Chobani, Under Armour, Microsoft, and many other well-known companies gained their first national exposure as honorees on the Inc. 5000.
“We’re on a mission to enable effective therapies to reach patients faster, and that requires us to build an enduring business,” said Dr. Michelle Longmire, CEO and co-founder of Medable. “We continue to focus on transforming clinical trial delivery and generating differentiated value for our customers and for patients. It’s a true honor to be among such great companies, and this recognition is testament to our outstanding team and our incredible customers.”
Medable has deployed its software-as-a-service platform in more than 300 decentralized and hybrid clinical trials in 60 countries, serving more than one million patients and research participants globally. Medable’s customers have achieved impressive results with decentralized and hybrid trials – including 200 percent faster enrollment and 50 percent cost reductions.
A 2022 financial modeling of decentralized trials using industry benchmark and Medable data and conducted by the Tufts Center for the Study of Drug Development shows that, on average, decentralized trials can achieve net financial benefits ranging from five to 13 times for Phase II and Phase III trials, due to reduced trial timelines and other factors.
The 2023 Inc. 5000 companies are ranked according to percentage revenue growth from 2019 to 2022. To qualify, companies must have been founded and generating revenue by March 31, 2019, and had to be U.S.-based, privately held, for-profit, and independent – not subsidiaries or divisions of other companies – as of December 31, 2022.
“Running a business has only gotten harder since the end of the pandemic,” says Inc. Editor-in-Chief Scott Omelianuk. “To make the Inc. 5000—with the fast growth that requires—is truly an accomplishment. Inc. is thrilled to honor the companies that are building our future.”
Complete results of the Inc. 5000, including company profiles and an interactive database that can be sorted by industry, metro area, and other criteria, can be found at the Inc. 5000 website.
About Medable
Medable is on a mission to get effective therapies to patients faster by transforming clinical drug development with disruptive technologies. The company’s digital platform streamlines design, recruitment, retention, and data quality for decentralized trials, replacing siloed systems with integrated digital tools, data and interfaces to accelerate trial execution. Medable connects patients, sites, and clinical trial teams to improve patient access, experience, and outcomes. Medable’s software has been named a Leader in the industry by both Everest Group and IDC. Medable is a privately held, venture-backed company headquartered in Palo Alto, California.
More about Inc . and the Inc. 5000
Methodology
Companies on the 2023 Inc. 5000 are ranked according to percentage revenue growth from 2019 to 2022. To qualify, companies must have been founded and generating revenue by March 31, 2019. They must be U.S.-based, privately held, for-profit, and independent—not subsidiaries or divisions of other companies—as of December 31, 2022. (Since then, some on the list may have gone public or been acquired.) The minimum revenue required for 2019 is $100,000; the minimum for 2022 is $2 million. As always, Inc. reserves the right to decline applicants for subjective reasons. Growth rates used to determine company rankings were calculated to four decimal places.
Inc. Business Media is the leading multimedia brand for entrepreneurs. Through its journalism, Inc. aims to inform, educate, and elevate the profile of our community: the risk-takers, the innovators, and the ultra-driven go-getters who are creating our future. Inc.’s award-winning work reaches more than 50 million people across a variety of channels, including events, print, digital, video, podcasts, newsletters, and social media. Its proprietary Inc. 5000 list, produced every year since 1982, analyzes company data to rank the fastest-growing privately held businesses in the United States. The recognition that comes with inclusion on this and other prestigious Inc. lists, such as Female Founders and Power Partners, gives the founders of top businesses the opportunity to engage with an exclusive community of their peers, and credibility that helps them drive sales and recruit talent. For more information, visit www.inc.com.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230817999027/en/
CONTACT: Lisa Barbadora, Big Valley for Medable
+1 (610) 420-3413
lbarbadora@bigvalley.co/media@medable.com
KEYWORD: CALIFORNIA UNITED STATES NORTH AMERICA
INDUSTRY KEYWORD: RESEARCH TECHNOLOGY CLINICAL TRIALS HEALTH TECHNOLOGY SOFTWARE BIOTECHNOLOGY PHARMACEUTICAL HEALTH DATA MANAGEMENT SCIENCE
SOURCE: Medable Inc.
Copyright Business Wire 2023.
PUB: 08/17/2023 07:37 AM/DISC: 08/17/2023 07:37 AM
http://www.businesswire.com/news/home/20230817999027/en